MercoPress. South Atlantic News Agency
Pfizer ready to deliver vaccine for kids under 5 before February's end, if okayed
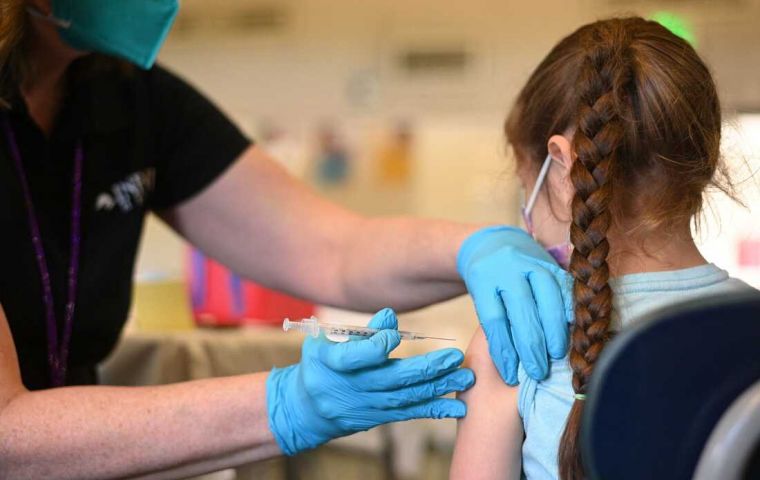 Almost 20 million children would be able to receive the shots, it was reported
Almost 20 million children would be able to receive the shots, it was reported Pharmaceutical giant Pfizer has announced it planned to submit a COVID-19 vaccine for children under the age of 5 to the Food and Drug Administration (FDA) of the United for emergency authorization. If approved, it would be the first such drug available in the United States for that age group.
Almost 20 million children would be able to receive the shots, it was reported.
Pfizer and partner BioNTech also said that, if clearance is given, doses would become available before the end of February. The laboratory also explained that as in the case of adults, this medication too needed to be administered in two separate injections. Pfizer's adult vaccine is currently authorized for everyone over the age of 5.
Late in 2021 Pfizer said its vaccine spurred an immune response in children between 6 months to 2 years old. It also found the drug was not as effective in children 2 to 4 years old, but still provided some protection.
According to health experts, Pfizer's child vaccine would represent a key tool in the fight against the Omicron COVID-19 variant, which has fueled cases worldwide.
On Monday, the FDA granted full approval to Moderna's vaccine, which will be marketed as Spikevax, for adults.
Meanwhile Novavax -already approved by Europe's EMA- has filed for FDA clearance. If endorsed, Novavax would be the fourth coronavirus vaccine available in the United States and also the first protein-based treatment to receive emergency US authorization.
The Novavax vaccine uses technology similar to the flu vaccine by introducing synthesized coronavirus proteins into the body, unlike mRNA drugs such as Pfizer's and Moderna's.
Novavax consists of two doses given 21 days apart and has already been approved by the European Commission and the World Health Organization.
“We're extremely proud of the work of our teams and we look forward to FDA's review of our EUA request,” Novavax CEO Stanley Erck said in a statement.
In December, the European Medicines Agency (EMA) had recommended approval for adults over age 18, citing recent trials conducted in Mexico, the United States and Britain.
Data from the Centers for Disease Control and Prevention (CDC) shows 211.7 million people in the United States are already considered fully vaccinated and 87.8 million have received a booster shot.
Health experts also hope the approval of the protein-based vaccine will persuade those who have been resistant to getting the mRNA shots to get immunized.




Top Comments
Disclaimer & comment rulesCommenting for this story is now closed.
If you have a Facebook account, become a fan and comment on our Facebook Page!