MercoPress. South Atlantic News Agency
Moderna says updated vaccine available to Uruguay has stronger antibody response
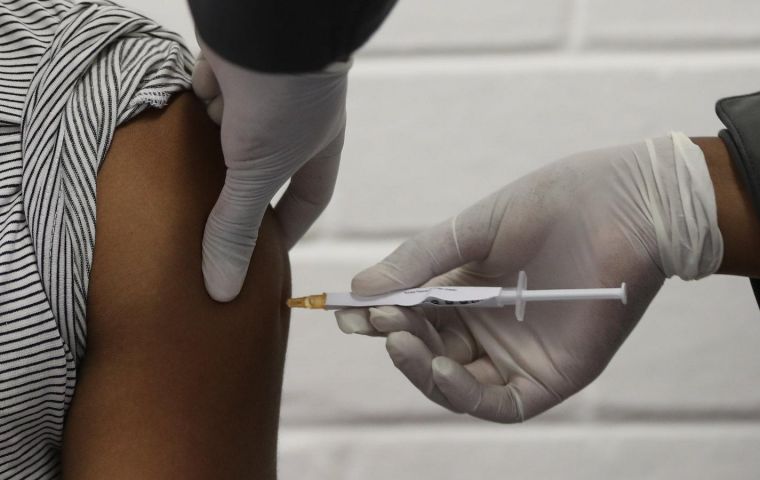 Moderna's Saglio confirmed the new booster would be available to the Uruguayan authorities for their Covid 2023 vaccination plan
Moderna's Saglio confirmed the new booster would be available to the Uruguayan authorities for their Covid 2023 vaccination plan The Moderna laboratories Tuesday reported that their two bivalent Omicron-targeting new messenger RNA (mRNA) technology booster vaccines (mRNA-1273.214 and mRNA-1273.222) have a superior antibody response compared to a booster dose of the basic RNA vaccine.
“We are pleased to see that our two bivalent booster vaccines offer superior protection against the Omicron BA.4/BA.5 variants compared to our original booster, which is encouraging given that COVID-19 remains a leading cause of hospitalization and death worldwide. In addition, the superior response against Omicron persisted for at least three months after the mRNA-1273.214 booster,” said Stéphane Bancel, CEO of Moderna.
Bancel's words came hours after Uruguay's Health Minister Daniel Salinas announced that the National Vaccine Advisory Committee had been summoned to assess the periodicity with which it will be necessary for people to be reinjected and “what type of vaccine” would this group recommend. Salinas insisted that whatever “the technicians and the scientists decide” will have full support from the authorities.
According to Moderna, the bivalent boosters also show, in research trials, neutralizing activity against BQ.1.1, an increasingly dominant emerging variant, confirming that updated vaccines have the potential to offer protection as the virus continues to rapidly evolve.
Moderna's Director of Commercial Alliances for Latin America, Roman Saglio, also confirmed that this booster is the one that the company will make available to the Uruguayan authorities for their Covid 2023 vaccination plan.
In an exploratory analysis of approximately 40 participants through research trials, both bivalent vaccines demonstrated strong neutralizing activity against BQ.1.1.
As published in the New England Journal of Medicine (NEJM), a 50 microgram booster dose of mRNA-1273.214 elicited a superior neutralizing antibody response against Omicron BA.1 and Omicron BA.4/BA.5 compared to the booster dose of mRNA-1273. In both sample groups, participants received the booster dose approximately 4.5 months after the previous vaccination.


Top Comments
Disclaimer & comment rulesCommenting for this story is now closed.
If you have a Facebook account, become a fan and comment on our Facebook Page!