MercoPress. South Atlantic News Agency
Relief for Paraguay: chicungunya vaccine in humans showed promising results
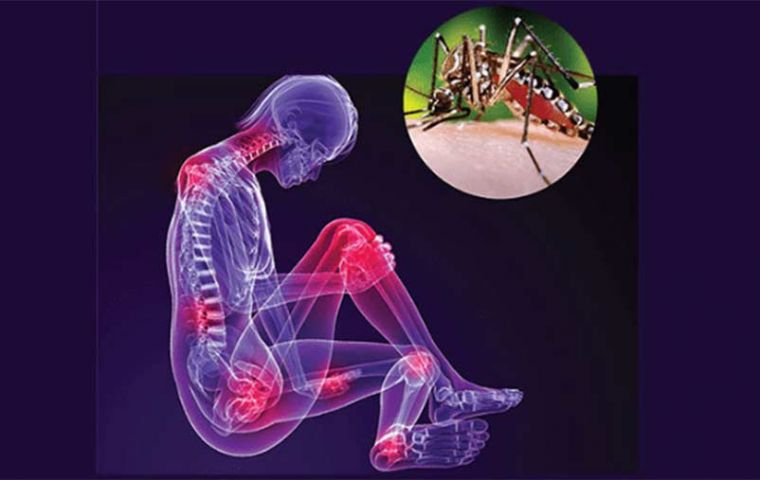 Chikungunya virus is transmitted by Anopheles mosquitoes.Tropical regions currently see the highest rates of the virus, with Paraguay, Brazil, Bolivia and Thailand most affected
Chikungunya virus is transmitted by Anopheles mosquitoes.Tropical regions currently see the highest rates of the virus, with Paraguay, Brazil, Bolivia and Thailand most affected A new clinical study shows promising results of a Phase III chikungunya vaccine trial, the first time the shot has been tested in humans. If approved by regulators, the vaccine would have the capacity to protect millions of people from the debilitating mosquito-borne disease, authors explained in the study published June 12 in The Lancet.
First discovered in Tanzania in the 1950s, the viral disease has since spread to various parts of Africa, Asia, the Caribbean and South America.
Symptoms include severe joint and muscle pain as well as high fever and skin rashes. There is currently no specific antiviral therapy. While symptoms usually improve within a couple of weeks, joint pain (arthralgia) can persist for months. In some cases, lasting arthralgia can lead to debilitating chronic rheumatic arthritis disease.
“Chikungunya is fatal in very few cases, but the disease is not pleasant. You can be sick for two weeks. In addition, in severe cases, you get very painful arthritis that can last for months,” said Peter Kremsner, a specialist in infectious and tropical diseases at Tübingen University in Germany.
Tropical regions currently see the highest rates of the virus, with Paraguay, Brazil, Bolivia and Thailand most affected. Global cases are relatively low, with Paraguay experiencing the most at 82,240 cases and 43 deaths between January and March 2023. Thailand saw 259 cases and no deaths in the period.
There are reports of chikungunya virus cases in African countries, but up-to-date infection data is difficult to verify. However, a major 2013 outbreak of chikungunya in South America led to over one million infections in just a few months. While death rates were low, around 52% of infected people experienced severe joint pain lasting months.
Studies suggest the disease caused the loss of 150,000 disability-adjusted life-years in 2014 alone. The measurement represents the loss of the equivalent of one year of full health.
The new Lancet study represents the results of the first Phase III trial conducted for a vaccine against the disease. According to the study, 28 days after a single vaccination with “VLA1553,” the vaccine resulted in virus-neutralizing antibody levels lasting up to 180 days in 98.9% of study participants.
“Chikungunya vaccination has shown very good efficacy in studies to date. After vaccination, a protective immune response can be detected in almost all vaccinated individuals after four weeks,” said Torsten Feldt, an infection and tropical disease specialist at University Hospital Düsseldorf, Germany.
The vaccine contains a modified, live version of the chikungunya virus that can replicate in the body without causing severe illness. Live vaccines closely mimic natural infections, triggering a robust immune response that provides long-lasting and broad protection.
They are very common — inoculations against measles, mumps and rubella (MMR combined vaccine), smallpox and yellow fever are all live vaccines. In 2018, the chikungunya virus was listed as a priority pathogen for vaccine development by the World Health Organization (WHO).




Top Comments
Disclaimer & comment rulesCommenting for this story is now closed.
If you have a Facebook account, become a fan and comment on our Facebook Page!