MercoPress. South Atlantic News Agency
Brazil remains mpox free after suspected case was ruled out
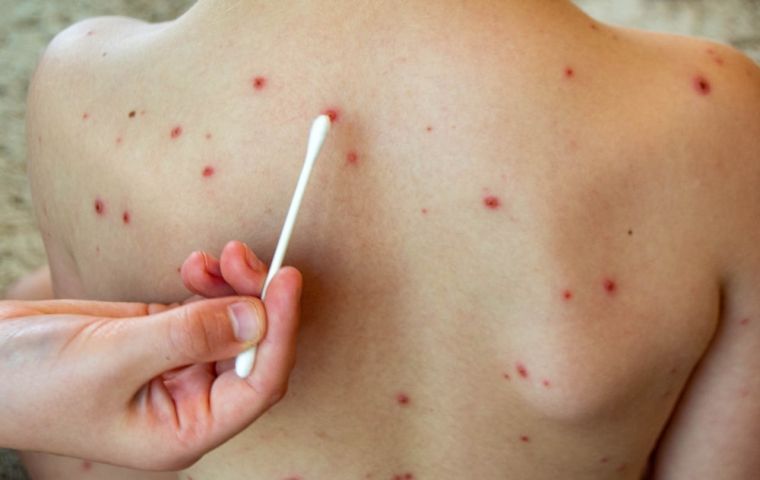 The patient was confirmed to have chickenpox though
The patient was confirmed to have chickenpox though A traveler landing Sunday at Sao Paulo's Guarulhos International Airport tested negative for mpox but positive for chickenpox, Agência Brasil reported Tuesday citing data from the São Paulo Health Department.
“The patient is fine, he is under observation. The case was ruled out for mpox and confirmed for chickenpox after an examination by the Adolfo Lutz Institute. It is important to note that the patient does not come from mpox endemic areas and that the care of patients with suspicion or disease diagnosis has been part of the institute's routine since 2022,” the government agency said in a statement after issuing an epidemiological mpox alert last Friday.
“The secretariat emphasizes that it is attentive to the epidemiological scenario and that all state health units already have technical recommendations for monitoring and following up the disease,” it added.
The document also highlighted that mpox transmission between humans occurs mainly through intimate contact with lesions on the skin or mucous membranes of infected people. The main symptoms of the disease are fever, weakness, swollen lymph nodes, muscle pain, back pain, headache, sore throat, nasal congestion, and cough.
Meanwhile, Brazil's National Health Surveillance Agency (Anvisa) renewed the registration exemption for the Jynneos vaccine and the Imvanex vaccine purchased by the Federal Health Ministry to prevent mpox. Anvisa's decision was published Tuesday in the Diário Official da Uniao (Official Gazette).
Anvisa's board of directors unanimously decided to authorize, on an exceptional and temporary basis, the renewal of the exemption from health registration of immunizers for a period of 180 days, starting August 23, 2024.
The Jynneos vaccine is manufactured by Bavarian Nordic in Denmark, while Imvanex is produced by IDT Biologika GmbH in Germany. According to Anvisa, both doses refer to the same product, with different nomenclature in the United States and Europe.
The Health Ministry has been brokering the emergency acquisition of 25,000 doses of mpox vaccines from the Pan American Health Organization (PAHO) after the malady was declared a public health emergency of international concern (PHEIC).
During the first global mpox emergency in 2023, Anvisa had already authorized the emergency use of the Jynneos vaccine, as the ingredient was not licensed in Brazil. The authorization was renewed in February this year but was to expire again this month.
The vaccine is intended for adults aged 18 years or over and has a shelf life of up to 60 months when stored at temperatures between -60 degrees Celsius (°C) and -40°C.




Top Comments
Disclaimer & comment rulesCommenting for this story is now closed.
If you have a Facebook account, become a fan and comment on our Facebook Page!