MercoPress. South Atlantic News Agency
Canada approves Moderna coronavirus vaccine: first batch of doses expected before the end of the year
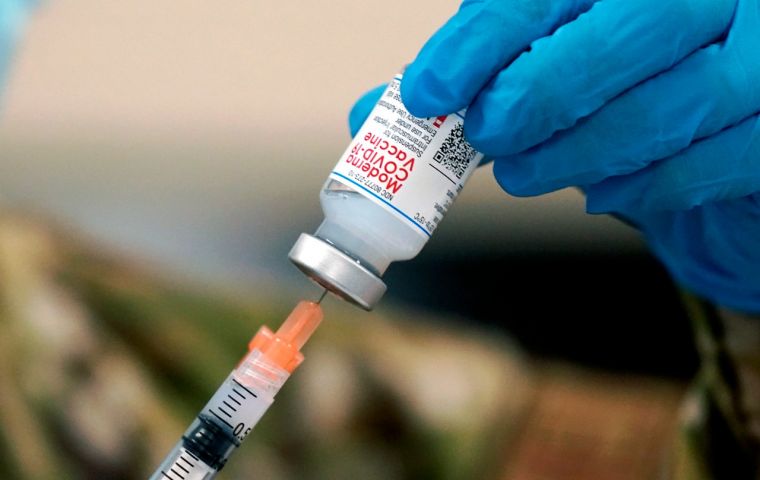 By the end of the year, Canada will receive up to 168,000 doses of the Moderna vaccine, which officials say will start to be administered in remote northern areas.
By the end of the year, Canada will receive up to 168,000 doses of the Moderna vaccine, which officials say will start to be administered in remote northern areas. Canada approved Moderna Inc's coronavirus vaccine on Wednesday, the second country to do so, paving the way for health authorities to step up an inoculation campaign against a worsening second wave.
By the end of the year, Canada will receive up to 168,000 doses of the Moderna vaccine, which officials say will start to be administered in remote northern areas.
By Jan. 31, Canada should have almost 1.2 million doses from both Moderna and Pfizer Inc, whose vaccine developed with German partner BioNTech SE was approved by Ottawa earlier this month. That figure will rise to 6 million by the end of March. Canada needs about 80 million doses in total.
“Know however dark the winter will be, spring is coming and better days will be back,” Prime Minister Justin Trudeau told a regular briefing.
Trudeau also said Canada would extend a 72-hour ban on passenger flights from Britain to Jan. 6, citing a new and more infectious variant of COVID-19. Many countries have introduced similar clampdowns.
Trudeau urged Canadians to stay at home, saying: “Even if you travel every winter, please rethink your plans ... the situation is very serious.”
A second wave of the novel coronavirus is sweeping Canada and medical officials in some parts of the country say the hospital system is under dangerous strain. Canada has recorded a total of 14,425 deaths and 521,509 cases.
“We're not out of the woods yet ... healthcare staff are exhausted,” Howard Njoo, Canada's deputy chief public health officer, told a separate briefing.
Authorities have begun administering the Pfizer/BioNTech vaccine to priority groups such as health workers and the elderly.
The United States approved the Moderna vaccine on Friday. It needs to be stored and shipped frozen but does not require the ultra-cold temperatures of the Pfizer/BioNTech shot.




Top Comments
Disclaimer & comment rulesCommenting for this story is now closed.
If you have a Facebook account, become a fan and comment on our Facebook Page!