MercoPress. South Atlantic News Agency
Brazil's Anvisa outlines differences between pediatric COVID-19 vaccines
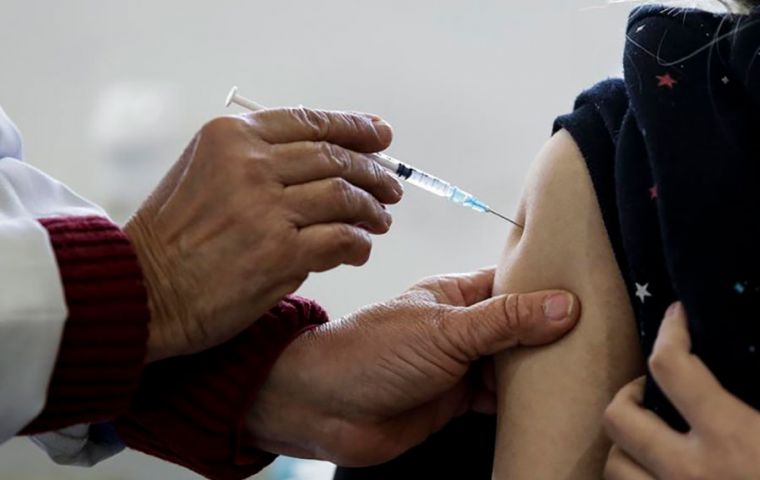 A batch of 1.8 million doses of Pfizer's pediatric vaccine will be arriving at Viracopos Airport Jan. 31
A batch of 1.8 million doses of Pfizer's pediatric vaccine will be arriving at Viracopos Airport Jan. 31 Brazil's National Health Surveillance Agency (Anvisa) Friday released a statement warning of the differences between COVID-19 vaccines for children to highlight the specifics of newly-released drugs.
The agency has authorized so far two brands of pediatric vaccines - Pfizer's, for children aged 5 to 11 years, and CoronaVac, produced in Sao Paulo by the Butantan Institute in an alliance with Chinese pharmaceutical company Sinovac, for patients between 6 and 17 years of age.
Pfizer's immunizer has a bottle with an orange cap and the dose is 0.2 ml, with 10 doses in each bottle. Preparation involves thawing the vials and diluting them with sodium chloride solution. Two doses should be administered, three weeks apart and the drug needs to be stored at a temperature between 2°C and 8°C for a period of up to ten weeks, without exceeding the validity period. The vaccine can also be frozen in -90°C to -60°C freezers, Anvisa explained.
The agency also pointed out that the CoronaVac vaccine can be used in both adults and children. The bottle has a gray cap, the dose is 0.5 ml and requires preparation by shaking the bottle before application, without diluting. Two doses are required with an interval of four weeks between injections. Storage should be carried out at temperatures from 2°C to 8°C the product's validty spans for 12 months.
Either brand is to be applied on the upper arm, the sanitary agency also pointed out.
Meanwhile, Pfizer announced a batch of 1.8 million doses of the pediatric vaccine against COVID-19 scheduled to be delivered Feb. 3 will be arriving at Viracopos Airport Jan. 31 instead, according to Health Ministry sources. Brazil has already received 4.2 million doses of the vaccine for children.
For patients over 12 years of age, 407.4 million doses have already been distributed, with 92% of the population in this age group (or 163.5 million Brazilians) having already received at least the first dose, while immunization with the second dose or a single-dose drug has already reached 150.9 million people, or 85% of the population over 12 years of age. In addition to that, 37.1 million people have already taken a booster dose, the Health Ministry also reported.




Top Comments
Disclaimer & comment rulesCommenting for this story is now closed.
If you have a Facebook account, become a fan and comment on our Facebook Page!