MercoPress. South Atlantic News Agency
Health & Science
-
Wednesday, March 31st 2021 - 04:06 UTC
World leaders seek international treaty under WHO for pandemics to come after covid-19

British Prime Minister Boris Johnson, German Chancellor Angela Merkel, and other top world leaders agreed Tuesday in a joint news article published in various top world newspapers that the international community should work together “towards a new international treaty for pandemic preparedness and response.” Also signing the declaration was World Health Organization (WHO) Chief Dr. Tedros Adhanom Ghebreyesus.
-
Tuesday, March 30th 2021 - 09:56 UTC
Lacalle gets vaccinated – insists on need for flexibility on the part of Mercosur
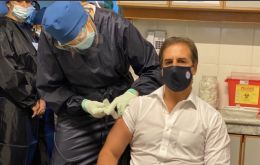
Uruguay's President Luis Lacalle Pou Monday stood by the words he said during Friday's virtual Mercosur Summit which led to a rife with his Argentina counterpart Alberto Fernández.
-
Tuesday, March 30th 2021 - 09:22 UTC
All but South African coronavirus strains circulating in Argentina's community

Argentina's Science Ministry Monday confirmed community circulation of the Manaus strain in the country had been established. The new variant is said to be more contagious and others also dare suggest it is even more lethal. Of the various Sars-CoV-2 strains known to science, only the South African one remains to be spotted in the country.
-
Tuesday, March 30th 2021 - 09:00 UTC
Florida Governor DeSantis against US vaccine passports

Florida State Governor Ron DeSantis Monday announced he would fight the federal administration's plans to create a national vaccine passport.
-
Tuesday, March 30th 2021 - 07:49 UTC
Total of covid-related deaths in London reaches zero

British health authorities Monday reported the number of deaths caused by any known variant of the covid-19 virus had amounted to zero, which was deemed a “fantastic milestone.”
-
Monday, March 29th 2021 - 10:18 UTC
Uruguay: Health system on the verge of saturation due to Covid-19

In Uruguay, 35.6% of operational intensive care beds (ICBs) are occupied by Covid-19 patients, reported the Uruguayan Society of Intensive Care Medicine (SUMI). Medical specialists warn that this is a limit that could lead to problems in the functioning of the ICBs.
-
Monday, March 29th 2021 - 10:03 UTC
Civil servants to work from home to halt circulation of people as Covid-19 cases rise in Argentina

Argentina's Cabinet Chief Santiago Cafiero and Health Minister Carla Vizzotti Sunday announced new measures in light of the increase in Covid-19 cases and fatalities as the Easter Week holidays loom over.
-
Monday, March 29th 2021 - 09:29 UTC
Piñera to seek postponement of April 10-11 elections in Chile

Chilean President Sebastián Piñera Sunday announced he would put forward a bill to postpone the April 10-11 elections until May in view of the country's critical situation regarding Covid-19 infections and diseases.
-
Monday, March 29th 2021 - 09:10 UTC
Astra-Zeneca vaccines arrive in Argentina

Over 200,000 doses of the University of Oxford /AstraZeneca covid-19 vaccine arrived in Buenos Aires Sunday through the Covax mechanism, which is promoted by the World Health Organization (WHO) and the Vaccine Alliance Gavi.
-
Monday, March 29th 2021 - 09:05 UTC
Biden bows to pressure – will promote US vaccine passport

The news came out Sunday in Washington that the administration of US President Joseph Biden was pushing for the development of a document whereby people should be available to prove whether they have been vaccinated against covid-19 or if they have the antibodies due to having had the disease, it was reported by The Washington Post.