MercoPress. South Atlantic News Agency
Health & Science
-
Monday, August 1st 2022 - 07:42 UTC
New Zealand fully reopens borders to build back from COVID isolation

The Government of New Zealand has fully reopened its borders effective August 1 to foreign visitors, thus ending the sanitary restrictions from the COVID-19 pandemic. The South Pacific country had one of the toughest travel regimes imposed on health grounds globally. Before Covid-19, tourism accounted for 5.5% of New Zealand's GDP.
-
Monday, August 1st 2022 - 07:06 UTC
Peru totals 282 cases of monkeypox
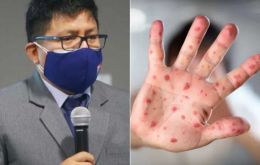
Peruvian health authorities have reported the number of monkeypox cases in the country had reached 282 Sunday, 34 days after the first infection was confirmed.
-
Monday, August 1st 2022 - 07:00 UTC
Biden tests positive again for COVID-19 after just a few days

US President Joseph Biden has tested positive again for COVID-19, the White House reported. According to his head physician, he “continues to feel well” and is asymptomatic but will nonetheless work from isolation.
-
Saturday, July 30th 2022 - 08:21 UTC
WHO concerned over hepatitis “of unknown origin” among children
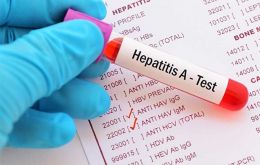
The World Health Organization (WHO) has expressed its concern over an outbreak of hepatitis “of unknown origin” among children. The agency also reported said it was working “side by side” with authorities in the affected countries to understand the cause of this infection.
-
Saturday, July 30th 2022 - 08:06 UTC
Uruguay has first case of monkeypox confirmed

Uruguayan health authorities Friday announced the first case of monkeypox had been confirmed in the country. According to the Ministry of Public Health (MSP), the infection was proved through PCR testing.
-
Friday, July 29th 2022 - 19:24 UTC
Brazil reports first death of patient with monkeypox
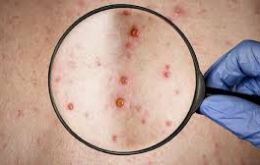
Brazilian health authorities Friday confirmed the first death linked to monkeypox in the country: a 41-year-old man, who was already under treatment for other diseases, including cancer, which caused the worsening of his health condition.
-
Friday, July 29th 2022 - 09:40 UTC
Argentine health authorities devise strategies against monkeypox

Argentine Health Minister Carla Vizzotti convened a meeting with “experts” to generate “strategies to strengthen epidemiological surveillance, work together with the jurisdictions to decentralize diagnosis, train health teams and generate information actions for society.”
-
Friday, July 29th 2022 - 09:39 UTC
Full dinosaur skeleton auctioned for over US$ 6 million

The complete skeleton of a dinosaur that lived at the end of the Cretaceous era has been sold Thursday in a Sotheby's auction in New York for US$ 6.1 million to an anonymous bidder who also purchased the rights to name the fossilized creature at will, it was announced.
-
Thursday, July 28th 2022 - 22:00 UTC
China: Full lockdown reinstated in Wuhan area due to COVID-19

In line with their Zero-COVID policy, Chinese authorities have placed an entire district on the outskirts of Wuhan under strict lockdown for the first time since 2020, it was reported.
-
Thursday, July 28th 2022 - 20:36 UTC
Uruguay back to vaccinating children against COVID-19
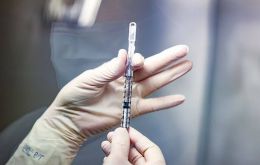
Uruguay Thursday resumed vaccinating children aged 5 to 13 against COVID-19 after an Appellate Court reversed earlier this week Judge Alejandro Recarey's ruling halting the therapeutic procedure.