MercoPress. South Atlantic News Agency
Tag: Anvisa
-
Wednesday, May 12th 2021 - 09:33 UTC
Brazil's Anvisa advises against AstraZeneca vaccines for pregnant women after one fatal case
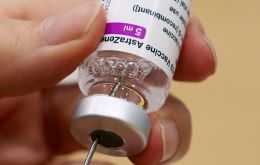
Brazil's Health Ministry Tuesday announced that vaccination of pregnant women and mothers in Brazil against Covid-19 will be restricted only to patients with comorbidities (pre-existing diseases) and they would only be given CoronaVac or Pfizer vaccines, following a death which might be linked to the AstraZeneca immunizer.
-
Friday, April 30th 2021 - 08:42 UTC
Sputnik V developers file libel suit against Anvisa for “false and inaccurate” rejection

The Russian manufacturers of the Sputnik V vaccine have filed a libel lawsuit in Brazil against the country's National Health Surveillance Agency (Anvisa) for “intentionally spreading false and inaccurate information” about the covid-19 inoculator after prohibiting its import, citing lack of data, the immunizer's Twitter account (@sputnikvaccine) reported.
-
Tuesday, April 27th 2021 - 09:42 UTC
Brazil's Anvisa decides against the Russian Sputnik V vaccine
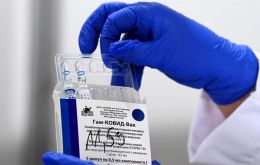
Brazil's National Health Surveillance Agency (Anvisa) Monday recommended against the import of the Russian-made Sputnik V vaccine to fight the covid-19 pandemic, citing lack of proper documents required for approval as well as other health risks the antidote may imply.
-
Monday, April 26th 2021 - 08:41 UTC
Anvisa expected to decide Monday on fate of Sputnik V vaccine in Brazil
Brazil's National Health Surveillance Agency (Anvisa) will evaluate this coming week the feasibility of importing the Russian-manufactured Sputnik V vaccine against covid-19.
-
Wednesday, April 21st 2021 - 09:35 UTC
Anvisa asks STF for more time to decide on the use of Sputnik V in Brazil
While Argentina has already started trials to produce the Russian Sputnik V vaccine against covid-19, Brazil’s National Health Surveillance Agency (Anvisa) has filed a petition before the Federal Supreme Court (STF) for additional time to evaluate a registration request for the very same immunization shot.
-
Tuesday, April 20th 2021 - 10:45 UTC
Brazil's Anvisa green-lights studies of new Chinese covid-19 vaccine

Brazil's National Health Surveillance Agency (Anvisa) has given the green light to clinical trials on volunteers of a potential anti-Covid-19 vaccine produced by the Chinese laboratory Sichuan Clover Biopharmaceuticals, it was announced.
-
Saturday, April 10th 2021 - 10:58 UTC
Brazil's Anvisa to check Russian Sputnik V manufacturing plant as need for vaccines soars
Brazil's National Health Surveillance Agency (Anvisa) will be sending a delegation to Russia next week to conduct an onsite review of the facilities where the Sputnik V vaccine is manufactured, it was announced Friday.
-
Friday, January 15th 2021 - 09:30 UTC
Delays in launching an inoculation program in Brazil, threatens the economic recovery
The risks to an expected economy recovery in Brazil during 2021 are increasing as delays in rolling out a vaccine program against coronavirus continues to be delayed, according to Moody's rating agency.
-
Monday, January 4th 2021 - 05:36 UTC
Brazil health regulator approves import of 2 million doses of AstraZeneca vaccine
Brazil's health regulator Anvisa said over the weekend it had approved the import of 2 million doses of the COVID-19 vaccine developed by AstraZeneca and the University of Oxford, although the jab is not yet approved for use in the country.
-
Wednesday, December 16th 2020 - 08:51 UTC
Brazil's health regulator ANVISA considers Chinese Covid-19 vaccine process non transparent
Brazil’s health regulator Anvisa said China’s health authorities are not transparent in their authorization of COVID-19 vaccines for emergency use, a statement that may further inflame political tension in the country.
