MercoPress. South Atlantic News Agency
Tag: Pfizer
-
Wednesday, April 6th 2022 - 09:00 UTC
COVID-19 booster shots are better if specific brands are used
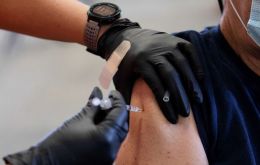
According to a study released Tuesday by Brazil's Oswaldo Cruz Foundation (Fiocruz), patients who have taken the two-shot scheme of CoronaVac's drug would be better off if they receive a booster injection of the Pfizer vaccine.
-
Tuesday, February 1st 2022 - 21:43 UTC
Pfizer ready to deliver vaccine for kids under 5 before February's end, if okayed
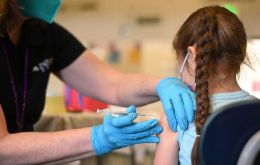
Pharmaceutical giant Pfizer has announced it planned to submit a COVID-19 vaccine for children under the age of 5 to the Food and Drug Administration (FDA) of the United for emergency authorization. If approved, it would be the first such drug available in the United States for that age group.
-
Saturday, January 29th 2022 - 09:23 UTC
Brazil's Anvisa outlines differences between pediatric COVID-19 vaccines

Brazil's National Health Surveillance Agency (Anvisa) Friday released a statement warning of the differences between COVID-19 vaccines for children to highlight the specifics of newly-released drugs.
-
Friday, January 28th 2022 - 09:11 UTC
European agency green-lights Pfizer COVID-19 pill

The European Medicines Agency (EMA) Thursday announced it had approved the use of Pfizer's anti-COVID-19 pill Paxlovid, which has thus become the first oral treatment cleared for use in the European Union. The new drug is a combination of the molecule PF-07321332 and the HIV antiviral Ritonavir.
-
Wednesday, December 22nd 2021 - 23:05 UTC
Uruguay, EU agree on reciprocity regarding digital vaccination certificates

Uruguay Tuesday became the fifth country to join the European Union's covid-19 certificate program. It is also South America's first.
-
Friday, December 3rd 2021 - 09:08 UTC
Former GACH members urge Uruguayans to take third doses of vaccine
Former members of Uruguay's Honorary Scientific Advisory Group (GACH) have recommended a third dose of vaccine against COVID-19 in addition to inoculating children aged 5 to 11.
-
Friday, November 26th 2021 - 08:46 UTC
Pfizer's vaccine makes it through to Canadian and European children
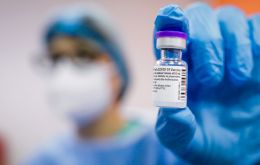
Canada Wednesday began vaccinating children aged 5 to 11 against COVID-19 in the French-speaking Quebec, while Ontario officially launched its campaign Thursday.
-
Saturday, November 13th 2021 - 09:11 UTC
Pfizer seeks clearance for Comirnaty to be used in Brazil on kids aged 5 to 11
The pharmaceutical company Pfizer Friday filed an application before Brazil's National Health Surveillance Agency (Anvisa) for its COVID-19 vaccine named Comirnaty to be cleared for use on patients aged 5 to 11 years old.
-
Saturday, November 6th 2021 - 09:44 UTC
US markets soar on the October jobs' report and Pfizer's announcement of an anti coronavirus pill

United States stocks closed the trading week at new records with NASDAQ hitting 16,000 points for the first time, following a strong jobs increase report from the US Labor Department.
-
Saturday, October 30th 2021 - 08:29 UTC
Pfizer vaccine gets emergency authorization for use on children aged 5 to 11 in the US
The Food and Drug Administration (FDA) of the United States Friday issued an emergency clearance for the use of the Pfizer / BioNTech vaccine against COVID-19 on children aged 5 to 11, it was announced. The decision came three days after a recommendation from a scientific committee.
